GMP certified rapid molecular testing
Producers of food and pharmaceutical products need to assure that their products are of high quality and not contaminated with any type of microorganism or DNA. This quality control testing has to be...

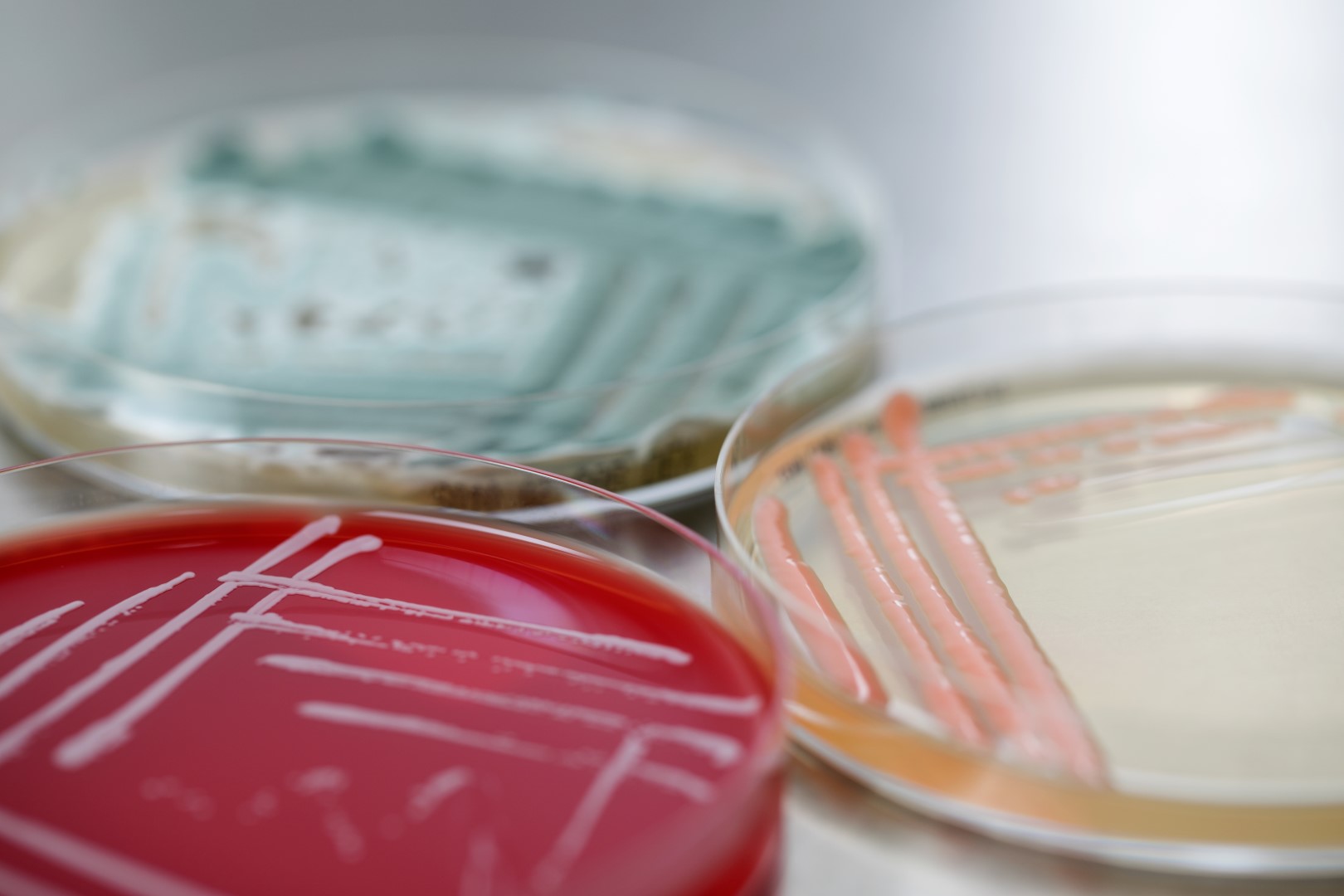
BaseClear offers a range of rapid microbial quality control services. With our GMP certified testing methods and decades of experience, we are a reliable and experienced partner for microbial quality control testing of food, biotechnological and pharmaceutical products. Our expertise and capabilities to process non-standard samples formats and deliver results with fast turnaround times are highly valued by our clients. Our services include microbial identification, root cause analysis using microbial strain typing, adventitious agents testing using next-gen sequencing, mycoplasma detection and cell line characterisation.
Our services will meet the quality level that you need and will fit perfectly within your quality system. You get the most reliable results that are in compliance with the global current Good Manufacturing Practice (GMP) as well as regulatory requirements and recommendations of the U.S. Food and Drug Administration (FDA) and the European Medicines Agency (EMA).
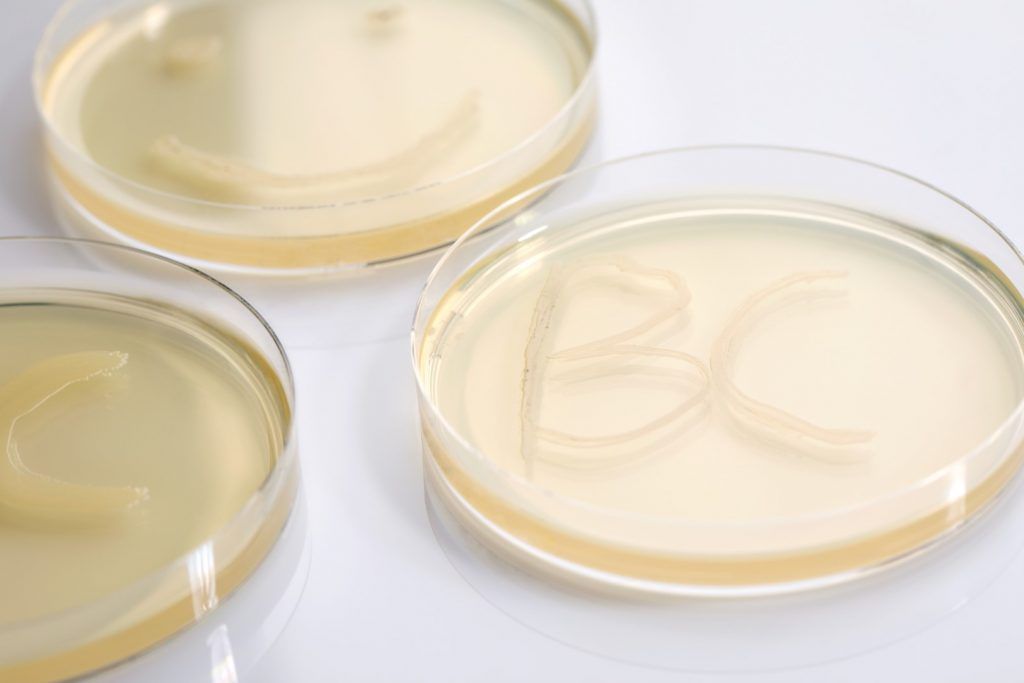

Producers of food and pharmaceutical products need to assure that their products are of high quality and not contaminated with any type of microorganism or DNA. This quality control testing has to be...

BaseClear offers testing services for very fast detection and identifications of microbial contaminants using state-of-the-art molecular methods. If necessary, we can deliver your microbial...

BaseClear’s rapid and validated contamination detection services ensure your cell cultures, virus stocks and final products are free from mycoplasma and other adventitious agents. Cell culture...
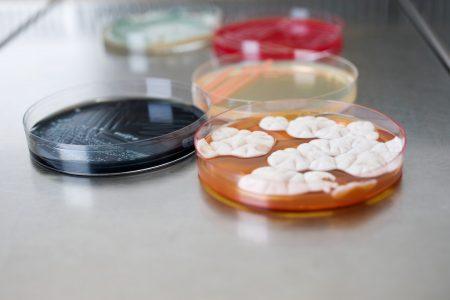
In case of a microbial contamination it is necessary to determine the root cause of the contamination problem. BaseClear offers all-inclusive microbial typing services for microbial root cause...
BaseClear is the outsourcing partner you are looking for. Do you need an expert who can help you regularly with the difficult samples you struggle with? BaseClear can do that for you. Are you looking for a back-up laboratory that can handle your samples in case of a temporary failure of your own identification systems, or do you have a temporary need for extra capacity? We are the right partner for you. Would you like to completely outsource your microbial identifications, having calculated that outsourcing could save you money? BaseClear indeed offers a full solution for identification and typing at the fastest delivery times and at competitive prices. Please contact us for more details!
For our production, it is important to check for microbiological contamination and to subsequently identify these on the basis of its DNA. BaseClear does excellent work and always sticks to the agreement made. I always receive a response within an hour.
In her role as Product Manager, Mubanga provides technical expertise and support to clients before, during and after custom projects relating to human health and other product lines. She collaborates within the team on customer projects and is involved with improving efficiency within the workflows. “I hold my work to a high standard because I’m passionate about what I do,” she comments.
Contact Mubanga